[ad_1]
A week after Apple held an event revealing its new iPhone, Apple Watch and AirPod models, the U.S. Food and Drug Administration has approved an Apple Watch function that can detect sleep apnea in device-wearers.
The sleep apnea detection tool comes four days before the launch date of Apple’s new Series 10 watch, which will be released on September 20. Those with existing Apple Watch Series 9 and Watch Ultra 2 models can use the sleep apnea feature starting today, however, with download of Apple’s newly released watchOS 11 software.
People who suffer from sleep apnea repeatedly stop and start breathing overnight and can, as a result, feel tired in the morning. Left undetected, sleep apnea can cause heart problems and other complications, in serious cases. Loud snoring can sometimes be a symptom. The condition affects an estimated 1 billion people worldwide and is often undiagnosed, according to health organizations including the American Heart Association.
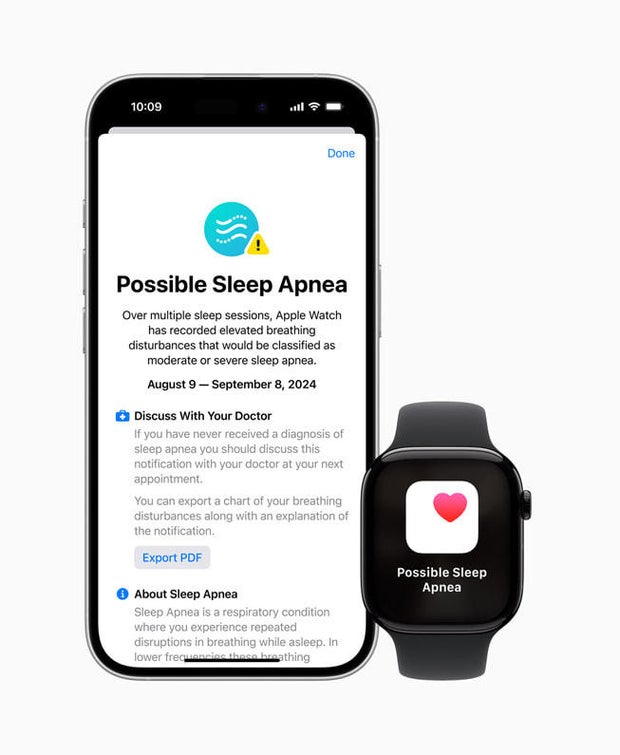
According to the FDA’s approval notice, the Apple Watch sleep apnea function is not a diagnostic tool, but rather an over-the-counter device designed to assess watch-wearers’ risk of sleep apnea. Apple says the new health feature, Breathing Disturbances, is a metric that acts as an “intelligent guardian for users’ health.”
The feature works using an accelerometer on the watch that detects “small movements at the wrist associated with interruptions to normal respiratory patterns” during sleep, according to Apple. Every 30 days, Apple Watch analyzes Breathing Disturbance data and alerts those users with a consistent pattern of sleep interruptions to visit a physician for possible diagnosis of sleep apnea.
Apple
“Empowering consumers everywhere to have the ability to reliably identify the presence of abnormal breathing patterns during sleep can help uncover a woefully underdiagnosed and serious medical condition such as sleep apnea,” Sairam Parthasarathy, professor of medicine at the University of Arizona and director of Health Sciences Center for Sleep, Circadian and Neurosciences Center for Sleep, UA Health Sciences, said in a statement. “This is a major step forward in improving public health.”
The FDA’s green-lighting of the sleep apnea feature comes on the heels of the agency’s approval of Apple’s AirPods Pro 2 as over-the-counter hearing aids for users with mild to moderate hearing loss, as the tech giant further cements itself as a player in the health tech market. Hearing experts praised the agency’s approval of the new earbuds as a positive for the OTC hearing market and for the roughly 30 million Americans who suffer from mild to moderate hearing loss.
[ad_2]
Source link